A privately held Israeli biotech startup has developed an electronic “pill” that delivers electronic stimulation to the digestive tract to suppress appetite.
Some 2.1 billion people — about 30 per cent of global population — are overweight or obese, and about 15 percent of healthcare costs in developed economies are driven by this condition.
More than a third of adult Americans are obese, and 29.1 million have diabetes, according to the Centers for Disease Control and Prevention. Both obesity and diabetes lead to higher health care spending, greater risk of health complications, and shorter life expectancy.
 The Israeli company, MelCap Systems, recognizes that many overweight or obese people are eager for a cost effective and safe way to help them be healthier and ease the potential risk of obesity.
The Israeli company, MelCap Systems, recognizes that many overweight or obese people are eager for a cost effective and safe way to help them be healthier and ease the potential risk of obesity.
The MelCap innovative system is based on a guided, ingestible autonomous wireless stimulating capsule with variable volume positioned in the human Gastrointestinal Tract (GI) and fixed at a targeted location by a magnetic system non-invasively attached to the body. The capsule is wirelessly controlled from outside.
Unlike other gastric stimulators on the market, the non-drug MelCap capsule is completely non-invasive and disposable, and claimed tom be 10 times less expensive than traditional, surgically implanted devices.
MelCap capsule is the size of a large pill and is swallowed by an overweight or obese person, arriving into the stomach. Once in the stomach, an external magnet is used to position the capsule most advantageously.
The MelCap system has 3 main parts:
• A remotely controlled capsule adapted for ingestion or placement into the gastrointestinal tract (GT) of a patient.
• A remote control device (which supports both iOS and Android devices) for controlling and sharing information with the capsule.
• A magnetic coupling system for fixing the capsule within the gastrointestinal tract of the patient.
Following ingestion of the capsule by the patient, it enters the GT. Location of the capsule within the GT is defined by an external magnetic field sensor, while the position of the capsule relative to the targeted nerve is determined by method of Nerve Conduction Study (NCS).
Once positioned in the desired location, the capsule is secured to an inner wall of the GT by the magnetic attraction between the magnetic element in the capsule and the magnetic element in the external positioning unit
The patient and/or medical personnel activate the capsule wirelessly by transmitting operating instructions to the capsule from the remote wireless control unit.
The capsule receives the operating instructions and configures and activates a pulse generator, contained in the capsule, to generate electrical pulses which propagate through the GT in proximity to at least one chosen area of GI tract. The pulses activate for instance a vagus nerve, thus enhancing gastric motility.
Depending on location of the capsule in the GT it can have different clinical effects: Gastric electrical stimulation (GES), Lower Esophagus Sphincter (LES) muscle simulation or vagus nerve blocking which require continuous monitoring and treatment.
MelCap has received a grant from The Office of the Chief Scientist (OCS) in the Israel Ministry of Industry, Trade and Labor, and the project has been examined and approved.
A first prototype mechanical design for the capsule has been completed, the electronic Concept is completed, and a magnetic fixation feasibility and proof of concept study was prepared and successfully validated. The company has applied for the IP protection, and recently got patent allowance from the US Patent Office, which claimed various designs of the capsule and the system.
The MelCap team includes:
MelCap CEO Mr. Josef More, Economics BA in Financing and Management, has been a seasonal entrepreneur, investor and founder of a number successful medical device companies. He belongs to the Alfred Mann Foundation, LIPOZOL ,(lipids for medical use), and MEDIRAD- intracoronary radiation. More has 25 years’ experience in managing and developing of five biotechnological projects, including three biomedical device projects and served for three years as director of Poalim High Tech.
Mr. Boris Vosnesensky, MelCap’s CTO, is an electric engineering professional, with more than 20 years of experience in medical devices field. Mr Vosnesensky is especially experienced in developing and manufacturing biomedical devices, and is an electronic engineering company owner — inventor and manufacturer of ELFcare electrotherapy device (http://www.mediseb.com)
MelCap Medical Director Prof. Abraham (Rami) Eliakim, head of the Gastroenterology Division in the Sheba Medical Center and Tel-Aviv University Faculty of Medicine, has more than 30 years of experience in Gastroenterology field, and is a world leader and pioneer in the field of capsule endoscopy. He consulted GIVEN IMAGING, acquired recently by Medtronic and Coviden in the development of the esophageal and colonic capsules.
Salk Institute for Biological Studies And The Imaginary Meal Pill
Taking a more conventional, but still innovative approach to weight control, researchers at the Salk Institute for Biological Studies in La Jolla, California have developed an innovative new type of weight loss pill that tricks the body into thinking it has consumed calories, inducing burning of body fat.
The compound’s formula effectively arrested weight gain, lowered cholesterol, controlled blood sugar and minimized inflammation in mouse models, making it a promising candidate for a rapid transition into human clinical trials.
Ronald Evans, director of Salk’s Gene Expression Laboratory, has developed a compound called fexaramine that acts like an imaginary meal. Fexaramine, which tricks the body into reacting as if it has consumed calories, and thus is a potentially effective obesity and diabetes treatment in humans.

Dr. Evans is an authority on hormones, both their normal activities and their roles in disease. A major achievement in the Evans lab was the discovery of a large family of molecules, named receptors, that respond to various steroid hormones, Vitamin A and thyroid hormones. These hormones help control sugar, salt, calcium and fat metabolism; thus, they impact on our daily health as well as treatment of disease. The receptors Evans discovered are primary targets in the treatment of breast cancer, prostate cancer and leukemia, as well as osteoporosis and asthma.
In addition, Dr. Evans’ studies led to a new hormone that appears to be the molecular trigger controlling the formation of fat cells. This hormone and its chemical derivatives represent one of the newest and most important advances in understanding problems arising from excess weight and obesity and as a potential treatment of adult onset diabetes (Type II diabetes) as well.
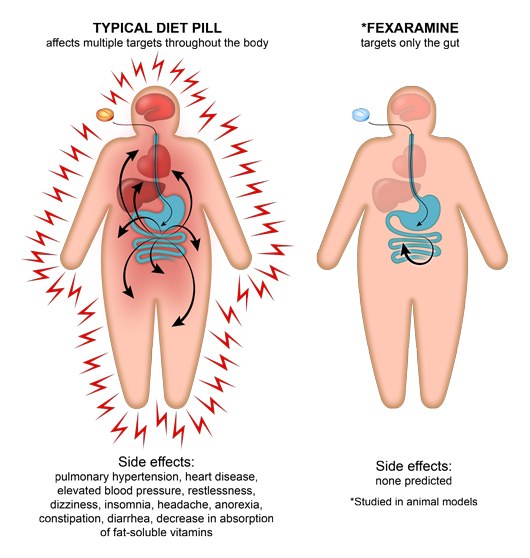
Unlike the active agents of most weight loss pills on the market, fexaramine doesn’t dissolve into the bloodstream like appetite suppressants or caffeine-based diet drugs, but remains in the intestines, causing fewer side effects.
 “This pill is like an imaginary meal,” says Dr. Evans, who is senior author of a paper recently published in the journal Nature Medicine. “It sends out the same signals that normally happen when you eat a lot of food, so the body starts clearing out space to store it. But there are no calories and no change in appetite.”
“This pill is like an imaginary meal,” says Dr. Evans, who is senior author of a paper recently published in the journal Nature Medicine. “It sends out the same signals that normally happen when you eat a lot of food, so the body starts clearing out space to store it. But there are no calories and no change in appetite.”
The Evans laboratory has spent nearly two decades studying the farensoid X receptor (FXR), a protein that plays a role in how the body releases bile acids from the liver, digests food and stores fats and sugars. The human body turns on FXR at the beginning of a meal, Dr. Evans’ team and other researchers have shown, to prepare for an influx of food. FXR not only triggers release of bile acids for digestion, but also changes blood sugar levels and causes the body to burn off some fats in preparation for the incoming meal.
Pharmaceutical companies researching treatments for obesity, diabetes, liver disease and other metabolic conditions have developed systemic drugs that activate FXR, turning on many pathways that FXR controls. Unfortunately, these drugs affect several organs and have unpleasant side effects, leading Dr. Evans to wonder whether switching on FXR only in the gut rather than in the intestines, liver, kidneys and adrenal glands all at once might yield a better outcome.
Salk researchers have demonstrated that fexaramine stops weight gain and burns fat in animal models. Since Fexaramine is only absorbed in the gut and does not enter the bloodstream, it isn’t associated with side effects common with typical diet pills. The researchers believe that pursuant to additional testing, their discovery will lead to effective weight loss and diabetes treatments for humans.
The Nature Medicine research paper, entitled “Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance” (Nature Medicine 21, 159–165 (2015) doi:10.1038/nm.3760), is coauthored by Sungsoon Fang, Jae Myoung Suh, Elizabeth Yu, Eiji Yoshihara, Sandra Jacinto, Yelizaveta Lukasheva, Annette R. Atkins, Ruth T. Yu, Michael Downes and Ronald M. Evans of the Gene Expression Laboratory, Salk Institute for Biological Studies, La Jolla, California; Shannon M Reilly and Alan R Saltiel of the Life Sciences Institute, University of Michigan, Ann Arbor, Michigan; Olivia Osborn, Denise Lackey, Bernd Schnabl, David A. Brenner and Jerrold M. Olefsky of the Department of Medicine, University of California San Diego; Alessia Perino and Kristina Schoonjans of Metabolic Signaling, Institute of Bioengineering, School of Life Sciences, Ecole Polytechnique Federale de Lausanne, Switzerland; Alexander Khvat of ChemDiv, Inc., San Diego, California; Sally Coulter & Christopher Liddle of the Storr Liver Unit, Westmead Millennium Institute, Sydney Medical School, University of Sydney, Australia
The work was supported by grants from the National Institutes of Health, Glenn Foundation for Medical Research, Leona M. and Harry B. Helmsley Charitable Trust, Ipsen Bioscience, California Institute for Regenerative Medicine, the Ellison Medical Foundation and the National Health and Medical Research Council of Australia. Dr. Ronald Evans also receives funding from the Howard Hughes Medical Institute.
The coauthors note that the systemic expression of the bile acid (BA) sensor farnesoid X receptor (FXR) has led to promising new therapies targeting cholesterol metabolism, triglyceride production, hepatic steatosis and biliary cholestasis. They note that in contrast to systemic therapy, bile acid release during a meal selectively activates intestinal FXR, and that by mimicking this tissue-selective effect, the gut-restricted FXR agonist fexaramine (Fex) robustly induces enteric fibroblast growth factor 15 (FGF15), leading to alterations in BA composition, but does so without activating FXR target genes in the liver. However, unlike systemic agonism, they find that Fex reduces diet-induced weight gain, body-wide inflammation and hepatic glucose production, while enhancing thermogenesis and browning of white adipose tissue (WAT). These pronounced metabolic improvements suggest tissue-restricted FXR activation as a new approach in the treatment of obesity and metabolic syndrome.
“When you eat, you have to quickly activate a series of responses all throughout the body,” says Dr. Evans. “And the reality is that the very first responder for all this is the intestine.”

“Dr. Evans and his colleagues developed the fexaramine compound by departing from the drug scaffold that most pharmaceutical companies typically pursue when targeting FXR. It turns out that when we administer this orally, it only acts in the gut,” explains Michael Downes, a Salk Institute for Biological Studies senior staff scientist and co-corresponding author of the Nature Medicine paper. “Giving one such drug in a daily pill form that only reaches the intestines — without transporting into the bloodstream that would carry the drug throughout the body — not only curtails side effects but also made the compound better at stopping weight gain.”
When the Salk scientists gave obese mice a fexaramine pill daily for five weeks, the mice stopped gaining weight, lost fat and had lower blood sugar and cholesterol levels than untreated mice. In addition, the mice had a rise in body temperature — which signals metabolism ramping up — and some deposits of white fat in their bodies converted into a healthier, energy-burning beige form of the tissue. Even collection of bacteria in the guts of mice shifted when they received the drug, although what those changes mean isn’t yet clear.
Why does fexaramine in the intestines work even better than drugs that simultaneously activate FXR throughout the body? Dr. Evans thinks it likely has to do with the natural order in which the body’s molecular pathways normally respond to a meal.
“The body’s response to a meal is like a relay race, and if you tell all the runners to go at the same time, you’ll never pass the baton,” says Dr. Evans. “We’ve learned how to trigger the first runner so that the rest of the events happen in a natural order.”
The Salk Institute for Biological Studies is one of the world’s preeminent basic research institutions. The Institute’s internationally renowned faculty probes fundamental life science questions in a unique, collaborative and creative environment. Focused both on discovery and on mentoring future generations of researchers, Salk scientists make contributions to our understanding of cancer, aging, Alzheimer’s disease, diabetes and infectious diseases by studying neuroscience, genetics, cell and plant biology and related disciplines.
Salk Institute faculty achievements have been recognized with numerous honors, including Nobel Prizes and memberships in the National Academy of Sciences. Founded in 1960 by polio vaccine pioneer Jonas Salk, MD, the Institute is an independent nonprofit organization and architectural landmark.
Sources:
MelCap Systems
Salk Institute for Biological Studies
Nature Medicine
Image Credits:
MelCap Systems
Salk Institute for Biological Studies

