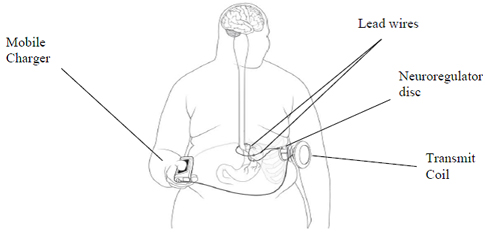
This week EnteroMedics, a medical device company that develops technology to treat obesity and metabolic diseases, finally received US Food and Drug Administration (FDA) approval for their Maestro® System; a decision they have been anticipating since May 2014. This is the first new medical device designed to treat obesity approved by the FDA in over a decade. The approval is an encouraging announcement for patients suffering from obesity and its related complications, who have also been unsuccessful at losing weight even after making life-style changes.
The Maestro System delivers EnteroMedics’ VBLOC® vagal blocking therapy that inhibits vagus nerve stimulation. This inhibition results in an increased feeling of fullness in the body, which ultimately reduces hunger. The Maestro System is laparoscopically implanted into the patient and uses high frequency, low-energy electrical pulses to block neural stimuli. The FDA approved this device after thoroughly evaluating the results from EnteroMedics’ ReCharge trial in which 233 patients were implanted with the device and monitored for a period of 24 months. The results showed that patients implanted with the device lost 8.5% more excess weight than the control group after 12 months.
For everyone at EnteroMedics associated with the trial, the results were encouraging and propelled the company to move forward in seeking FDA approval. As expressed by EnteroMedics’ Chief Consulting Medical Officer on the project Scott Shikora, MD, FACS, “VBLOC Therapy offers an entirely new approach to the treatment of obesity. By blocking signals along the nerves that connect the brain and stomach, VBLOC reduces feelings of hunger and promotes earlier feelings of fullness, which can help people with obesity reduce the number of calories consumed and promote safe, healthy and durable weight loss.”
FDA approval is not only an important announcement for obese patients; it is also a step towards decreasing health costs and reducing premiums taxpayers pay to subsidize the added medical charges incurred due to this condition. Obesity rates in the U.S. are currently at epidemic levels and are strongly associated with severe health and economic impacts. It has been said that obesity now costs Americans more in healthcare spending than smoking. In 2010, the Congressional Budget Office reported that nearly 20 percent of the increase in U.S. health care spending (from 1987-2007) was caused by obesity. Annual health costs related to obesity in the U.S. comes with a nearly $200 billion price tag, and nearly 21 percent of medical costs in the U.S. can be attributed to obesity.
The Maestro System is approved for obese patients aged 18 to 65, with a body mass index (BMI) of 40 to 45 or, a BMI of 35 to 39.9 and a related health condition such as high blood pressure or high cholesterol. Such patients should also have tried to lose weight, through diet and exercise, at least once in the last five years.
The company’s excitement over the approval is reflected in a statement made by Mark B. Knudson, Ph.D., EnteroMedics’ President and Chief Executive Officer, “FDA approval of VBLOC Therapy is a transformational event for not only EnteroMedics and the many supporters who have helped us achieve this milestone but, more importantly, for the people with the diseases of obesity that have been waiting for a new option. The Maestro System fills a significant gap in the currently available treatment options, offering clinically meaningful weight loss without the fear or many of the side effects associated with existing bariatric options.” This approval is not the final step in the process and it is contingent on evidence that EnteroMedics will provide the FDA over the next five years.


