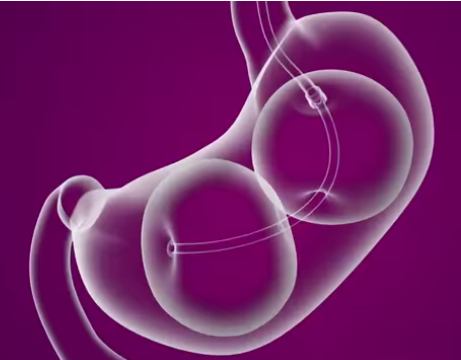
The U.S. Food and Drug Administration has granted approval of a new inflatable intragastric balloon device to treat obesity that requires no invasive surgery to install and remove. The ReShape Integrated Dual Balloon System (ReShape Dual Balloon) is designed to induce weight loss in obese adult patients. It is thought to work either by occupying space in the stomach thereby imparting feelings of fullness, or possibly by other mechanisms not yet understood.
The ReShape Dual Balloon System can be placed in a patient’s stomach in less than half an hour in a minimally invasive endoscopic outpatient procedure during which the patient is put under conscious sedation, so instead of a general anesthetic that causes unconsciousness, the patient stays somewhat aware of his or her surroundings but doesn’t feel pain or anxiety and can go home on the same day as the procedure.
A small camera (an endoscope) is guided into the stomach through the mouth and helps guide placement of the un-inflated dual balloons. Once placed, the balloons are carefully filled with a sterile saline (salt water) and will stay in place for six months.
The ReShape Procedure offers an alternative to surgery in which the subject’s stomach is not either surgically made smaller or fitted with a gastric band. Instead the dual balloon fills up the stomach in place of food. The dual balloon is also different to single balloons in that it is designed to fit the natural shape of the stomach, helping the patient feel full without over-stretching the stomach to minimize discomfort. The total volume of both balloons is 900 ml of saline — more volume than single balloons, which typically have a volume of 400-700 ml in one balloon.
Unlike surgery which involves general anesthesia, incisions, stitches and scarring the balloons don’t alter the natural size or shape of the stomach, but just physically occupy space that food might normally fill. After six months, via a similar outpatient procedure to the one described above, the ReShape balloons are carefully drained and the deflated dual balloon retrieved through the mouth.
Patients are advised to follow a medically supervised diet and exercise plan to augment their weight loss efforts while using the ReShape Dual Balloon and to maintain their weight loss following its removal.
In terms of effectiveness and safety: each balloon is filled and sealed independently, so in the unlikely instance of a balloon deflating in situ, the other balloon will hold the device in place until it can be safely removed.
The ReShape Dual Balloon was studied in a clinical trial with 326 obese participants aged 22 to 60 (with a BMI of 30 kg/m2 to 40 kg/m2) who had at least one obesity-related health condition. During a clinical study, a group of people who used this device lost more weight than those who did not use it. The study included a total of 326 patients at 8 investigational sites in the United States. Of the 326 patients, 187 received the device and 139 underwent the endoscopic procedure but did not receive the device.
In the study, 187 individuals randomly selected to receive the ReShape Dual Balloon lost 14.3 pounds on average (6.8 percent of their total body weight) when the device was removed at six months, while the control group (who underwent an endoscopic procedure but were not given the device) lost an average of 7.2 pounds (3.3 percent of their total body weight). All study participants received diet and exercise counseling.
Six months following the device removal, patients treated with the ReShape Dual Balloon device kept off an average of 9.9 pounds of the 14.3 pounds they lost.
The ReShape Integrated Dual Balloon System was developed by San Clemente, California based ReShape Medical, which specializes in the ReShape Dual Balloon System as the first and only dual-intragastric balloon for weight loss. The Reshape Balloon is manufactured in the USA, has been available in Europe since 2007 and carries a CE mark, which is the manufacturer’s declaration that the product meets the requirements of applicable European Community directives.
“For those with obesity, significant weight loss and maintenance of that weight loss often requires a combination of solutions including efforts to improve diet and exercise habits,” says William Maisel, M.D., M.P.H., acting director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health. “This new balloon device provides doctors and patients with a new non-surgical option that can be quickly implanted, is non-permanent, and can be easily removed.”
The ReShape Dual Balloon is indicated for weight reduction in obese adult patients with a body mass index (BMI) of 30 to 40 kg/m2. The device is limited to patients with one or more obesity-related conditions such as high blood pressure, high cholesterol, and diabetes, and is intended for patients who have failed previous attempts at weight loss through diet and exercise alone.
Potential side effects that have been associated with the procedure include headache, muscle pain, and nausea from the sedation and procedure; in rare cases, severe allergic reaction, heart attack, esophageal tear, infection, and breathing difficulties can occur. Once the device is placed in the stomach, patients may experience vomiting, nausea, abdominal pain, gastric ulcers, and feelings of indigestion.
This device should not be used in patients who have had previous gastrointestinal or bariatric surgery or who have been diagnosed with inflammatory intestinal or bowel disease, large hiatal hernia, symptoms of delayed gastric emptying or active H. Pylori infection; those who are pregnant or use aspirin daily should also avoid the device.
There are currently three other FDA-approved devices to treat morbid obesity: the Allergan LAP-Band, the Ethicon Endo-Surgery Realize Adjustable Gastric Band and the Maestro Rechargeable System.
Sources:
The U.S. Food and Drug Administration (FDA)
ReShape Medical
Image Credits:
ReShape Medical
William Maisel