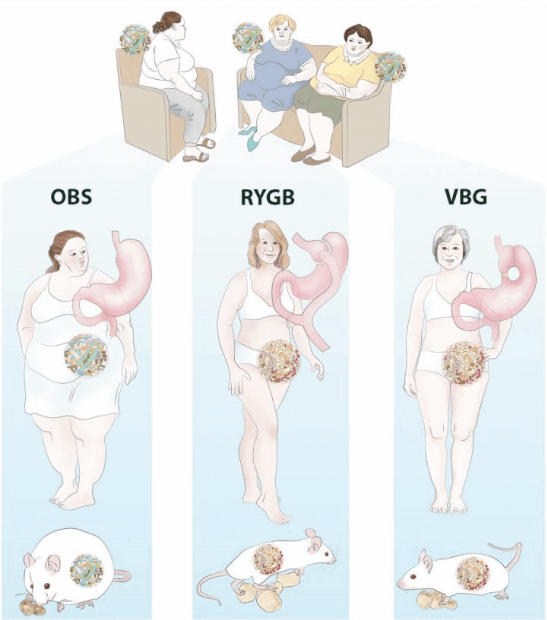
Results of a new human study published in the August 4 edition of the journal Cell Metabolism show that two bariatric surgery methods: Roux-en-Y gastric bypass (RYGB) and vertical banded gastroplasty (VBG), result in similar changes to the gut microbiome that are maintained a decade later in a group of female trial participants. The researchers observe that transfer of microbiota from the bariatric surgery patients was shown to decrease fat mass and increase carbohydrate utilization in mice.
The article notes that weight loss surgeries in patients have previously been associated with altered gut microbes, with how long these microbiome changes last and whether they are directly associated with weight loss not known.
However, the Cell Metabolism paper shows that both of the referenced bariatric surgery procedures result in similar microbiome composition changes, and that that these changes are maintained a decade later.
Moreover, the researchers determined that the microbiome changes are specific to the surgery and not just a reflection of altered weight changes (BMI), which makes exploration of probiotics as an alternative to weight-loss surgery a reasonable, science-based proposition.
In a 2011 paper published in the journal Trends In Endocrinology and Metabolism entitled “Effects of the gut microbiota on obesity and glucose homeostasis“ (Volume 22, Issue 4, April 2011, Pages 117–123 ) Thomas Greiner, Fredrik Bäckhed of the Sahlgrenska Center for Cardiovascular and Metabolic Research/Wallenberg Laboratory at the University of Gothenburg Department of Molecular and Clinical Medicinein Gothenburg, Sweden note that the human gut is home to a vast number of bacteria — the microbiota — whose genomes complement our own human sets of genes. Drs. Greiner and Bäckhed observe that the gut microbiota function at the intersection between host genotype and diet in order to modulate host physiology and metabolism, observing that recent data have revealed that gut microbiota can affect obesity, contributing to host metabolism by several mechanisms including increased energy harvest from the diet, modulation of lipid metabolism, altered endocrine function, and increased inflammatory tone. Therefore, they conclude that gut microbiota could be considered an environmental factor that modulates obesity and other metabolic diseases.
A new Clinical & Translational Report published August 4 in the journal Cell Metabolism entitled “Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation“ (Cell Metabolism, August 2015 DOI: 10.1016/j.cmet.2015.07.009), coauthored by Valentina Tremaroli, Fredrik Karlsson, Malin Werling, Carel W. le Roux, Jens Nielsen, Petia Kovatcheva-Datchary, Torsten Olbers, Lars Fandriks and Fredrik Bäckhed, finds that RYGB and VBG induce long-term alterations in the human gut microbiome and these changes are not BMI dependent. They observe that RYGB and VBG have different effects on bile acid and Trimethylamine N-oxide (TMAO) metabolism, and produce long-term alterations of the gut microbiome independently of BMI, that these alterations modulate host metabolism and fat mass deposition and the surgically altered microbiome contributes to fat mass regulation.
The report’s coauthors observe that prevalence of obesity and its metabolic comorbidities, such as type 2 diabetes and cardiovascular disease are on the increase worldwide, and although several contributory genetic and environmental factors have been identified, the mechanisms responsible for excess fat mass accumulation and development of obesity are not well understood.
However, recent research shows that intestinal microbes contribute to the regulation of energy homeostasis and fat storage and this may play a role in development of obesity and its complications, noting that obesity has been associated with altered gut microbiota composition, reduced microbial diversity, and reduced gene richness. Additionally, dietary weight loss interventions have been shown to increase gene richness of gut microbiota and to shift its composition toward that found in lean individuals.
Bariatric surgery, which shrinks stomach volume, has become increasingly popular as an efficient and relatively safe means of losing weight and is currently considered the best treatment for sustained weight loss and reduction of obesity-related comorbidities and the most effective procedure for treatment of obesity, Given the role gut microbiota play in regulating host metabolism and adiposity, the researchers investigated long-term effects of bariatric surgery on the microbiome of patients randomized to Roux-en-Y gastric bypass or vertical banded gastroplasty and matched for weight and fat mass loss.
Why and how bariatric surgery works is still somewhat mysterious, but it has been apparently able to benefit obese individuals in more ways than simply shedding pounds — such as by improving or even resolving conditions such as type 2 diabetes and hypertension with changes to the gut microbiome seeming to play an important role in metabolic benefits gained from bariatric surgery. However the question has been how long these benefits would last.
To investigate, Dr. Bäckhed and his colleagues examined gut bacteria of 14 women over a period of nine years after they had undergone Roux-en-Y gastric bypass or vertical banded gastroplasty. Despite respective differences in the two surgery procedures, both had similarly long-lasting changes on the gut microbiome with the effects shown to be transferable in murine tests in which “germ-free” mice (specially bred to be free of all gut bacteria) were treated with stool samples from patients who had undergone bariatric surgery. Mice receiving the stool transplants were able to better metabolize fat via oxidation or breakdown and put on significantly less fat compared to mice colonized with stool from obese individuals.
Dr. Bäckhed, who is an expert in cellular microbiology and mouse physiology, combines clinical oriented research with gnotobiotic mouse models to address the role of the normal gut microbiota in metabolic diseases. “Our findings are important in light of the growing epidemic of obesity and associated diseases,” says Dr Bäckhed in a Cell Press release. “Since surgery always confers a risk, it is critical to identify non-surgical strategies. One potential strategy would be to devise novel probiotics based on our findings that can be supplied to obese individuals.”
Sources:
Cell Press
Trends In Endocrinology and Metabolism
Cell Metabolism
Image Credits:
Tremaroli and Karlsson et al./Cell Metabolism 2015
The University of Gothenburg

