Austin, Texas based medical device firm Apollo Endosurgery, Inc. reports that its latest minimally-invasive endoscopic surgical product for bariatric and gastrointestinal procedures — the ORBERA gastric balloon, has received approval from the U.S. Food and Drug Administration (FDA) as a weight-loss system for adult patients suffering from obesity — ie: persons with a body mass index (BMI) of 30-40 kg/m2, and have been unable to lose weight through diet and exercise — in shedding pounds and maintaining a healthier BMI.
The ORBERA balloon is part of Apollo Endosurgery, Inc.’s ORBERA Managed Weight Loss System, a comprehensive, non-surgical two-part program that includes insertion of a balloon for filling space in a patient’s stomach as an aid to proper portion control. Data on ORBERA collected in a U.S. clinical trial has demonstrated that the average trial participant lost 3.1 times the amount of weight with the ORBERA balloon in place as compared with trial subjects who relied on diet and exercise alone over an interval of six months.
ORBERA is an incision-less, non-surgical weight loss solution designed for adult patients suffering from obesity, who are not appropriate for or considering invasive surgery, but for whom diet and exercise or pharmaceutical interventions have not worked.
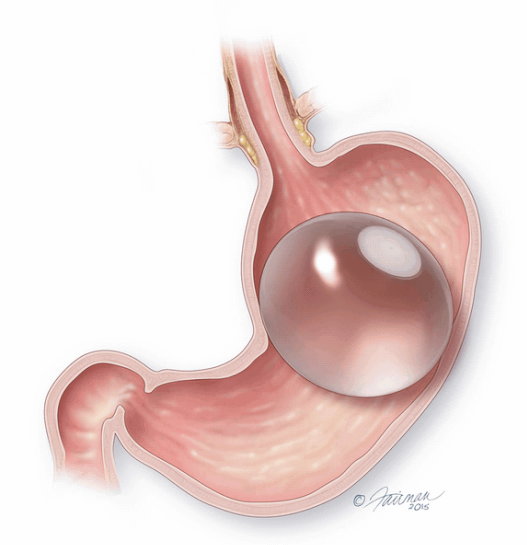
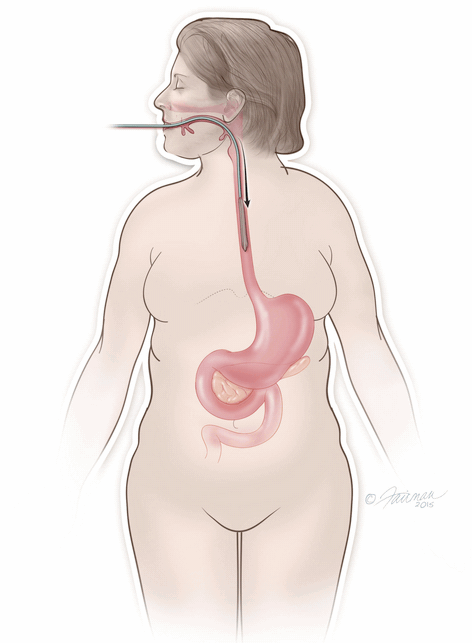
The ORBERA Intragastric Balloon System’s gastric balloon component is placed in the stomach through the mouth, by way of a minimally invasive endoscopic procedure, with the patient under mild sedation.
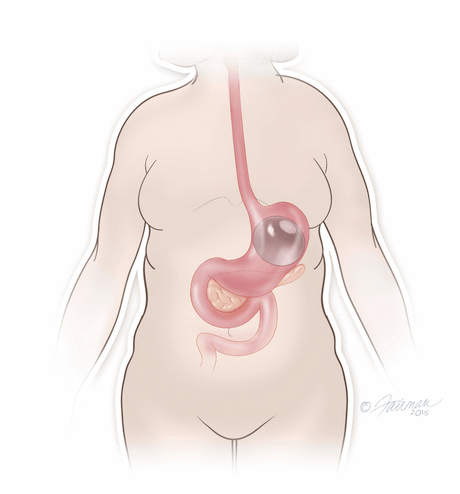
Once in place, the thin and deflated ORBERA balloon is then filled with salt water (saline solution) until it assumes a spherical shape about the size of a grapefruit. The balloon can be filled with different amounts of saline (from 400 to 700 cc) to best match an individual patient’s body size and characteristics.The procedure typically takes about 20 to 30 minutes and the patient can generally go home the same day.
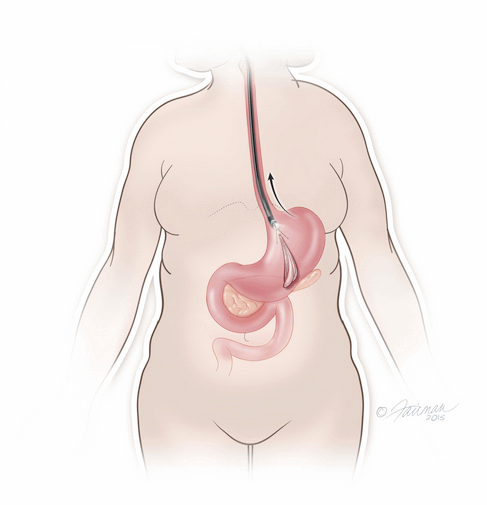
The system is temporary and should be removed after 6 months through another non-surgical procedure done under a mild sedative, in which the ORBERA balloon is deflated and then extracted.
ORBERA is intended to be used while a patient participates in a diet and exercise plan supervised by a health care provider.
During the time when the balloon is in place, the patient receives an individually tailored support program through the ORBERA Managed Weight Loss System team of experts — which may include a dietician, psychologist and exercise physiologist — to help keep them motivated, coordinate their program and help work through weight loss barriers preventing them from achieving their long-term weight loss goals. Coaching continues over 12 months, even though the balloon is removed after six months. The program is designed to help the patient develop sustainable, healthy habits that will help them maintain an healthier weight over time.
The FDA reports that during the U.S. pivotal ORBERA clinical trial, which was a multi-center, prospective, randomized, non-blinded comparative study, the group of participants who used the ORBERA Intragastric Balloon lost more weight than controls who did not. The study included a total of 255 patients who were followed for one-year, during which 125 patients received the device, which remained in place for six months. They concurrently participated in a 12-month behavioral modification program. Another control group of 130 patients participated in a 12-month behavior modification program alone, but did not receive the ORBERA device. After the device was removed, the subjects were followed for an additional six months with regular office visits continuing through a total of one year.
Patients with ORBERA lost an average of 21.8 pounds (10.2% of their body weight) after the device had been in place for six months. Three months after the device was removed (9 months after device placement), patients who received the device were able to maintain an average of 19.4 pounds in weight reduction. The 130 patients who participated in the behavior modification program but did not receive the device lost only an average of 7.0 pounds (3.3% of their body weight).
For more than 20 years, the global medical community has been using intragastric balloons from the makers of ORBERA to help thousands of people lose weight.
“While new to the United States, ORBERA is a weight loss device with more than 220,000 balloons distributed in over 80 countries and approximately 230 published papers documenting its clinical results,” says Apollo Endosurgery Chief Executive Officer Todd Newton. “ORBERA is a proven, innovative and non-surgical solution to help fight the obesity epidemic and treat patients before their disease progresses and requires more invasive treatments. With the FDA approval of ORBERA, Apollo can now offer this safe and effective solution to patients and their physicians in the United States.”
“For many, the weight loss journey leaves patients with little support or options other than diet and exercise and traditional surgery. The approval of ORBERA fills this gap in available treatments and is an exciting development for healthcare professionals who are committed to providing patients with less invasive options that can assist them in reaching their long-term weight loss goals,” observes New York-based bariatric surgeon Christine Ren-Fielding, M.D.. “ORBERA gives us a new weight loss option to help address what has become a critical health issue in the United States.”
Detailed findings from the clinical trial include:
• At month six, the ORBERA group achieved a mean of 38.4 percent Excess Weight Loss (EWL).
• Mean Total Body Weight Loss (TBWL) at six months was 10.2 percent for the treatment group compared to 3.3 percent TBWL for the control group.
• The ORBERA group lost 3.1 times as much weight as the control group at six months.
• The ORBERA group also lost significantly more weight than the control group over the course of the study, and was able to maintain significant weight loss through month 12, six months after removal of the device.
Counteridications:
The FDA says the ORBERA device should not be used in patients who:
• Already have an intragastric balloon
• Have had prior gastrointestinal or bariatric surgery
• Have any inflammatory disease of the gastrointestinal tract
• Have potential upper gastrointestinal bleeding conditions
• Have a large hiatal hernia
• Have a structural abnormality in the esophagus or pharynx
• Have serious esophageal motility disorders
• Have a gastric mass
• Have a severe clotting or bleeding disorder (coagulopathy)
• Have liver failure (hepatic insufficiency) or cirrhosis
• Are known to have or suspected to have an allergic reaction to have any other medical condition which would not permit elective endoscopy
• Have a serious prior or present psychiatric illness
• Have alcoholism or drug addiction
• Who are unable or unwilling to take prescribed proton pump inhibitor medication for the duration of the device implant
• Are unable or unwilling to participate in a medically supervised diet and behavior modification program with routine medical follow-up
are receiving aspirin, anti-inflammatory agents, blood thinners (anticoagulants) or other gastric irritants
• Are pregnant or breast-feeding
For full safety information visit http://orbera.com/dfu, talk with your doctor, or call Apollo Customer Support at 1-855-MYORBERA.
Apollo Endosurgery, Inc.’s bariatric products fill the gap between non-surgical weight loss solutions and invasive surgeries, allowing physicians to do more for those patients who require more than drug therapy and dietary advice. Other Apollo products include the OverStitch Endoscopic Suturing System that facilitates full thickness surgical suturing delivered through a flexible endoscope, and the FDA-approved LAP-BAND Adjustable Gastric Banding System weight loss device. Apollo is a bringing new technologies and innovative products to markets in over 80 countries today. More information regarding Apollo Endosurgery can be found at: http://www.apolloendo.com
Sources:
Apollo Endosurgery, Inc.
U.S. Food and Drug Administration (FDA)
Image Credits:
Apollo Endosurgery, Inc.